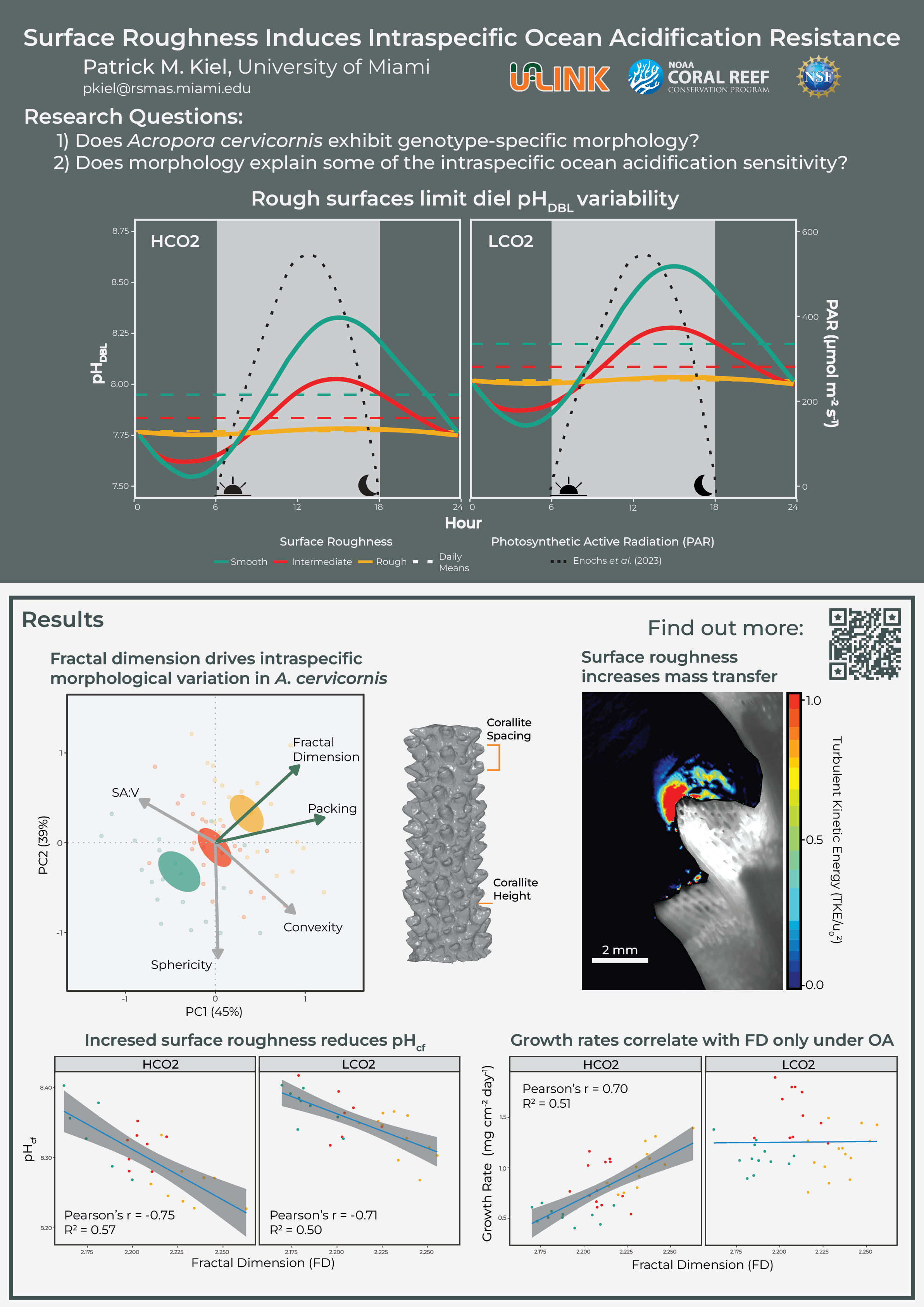
Surface Roughness Induces Intraspecific Ocean Acidification Resistance
research 11 May 2024Thank you for checking out my poster at the Microscale Ocean Biophysics Meeting 7.0 at Heron Island, Australia. My poster is titled, “Surface Roughness Induces Intraspecific Ocean Acidification Resistance”. This work was made possible by my co-authors and supervisors including Vivek Prakash, Diego Lirman, Ian Enochs, Prannoy Suraneni, Arash Sharifi, Amanda Oehlert, Ali Pourmand, Nash Soderberg, and Albert Boyd.
Overview
Coral restoration efforts are expanding globally to enhance gene flow and coral cover on degraded reefs, aiming to buy time while climate change and poor water quality are systematically addressed (Suggett et al. 2024). These restoration programs have produced large-scale coral nurseries where hundreds of genetically distinct individuals (“genets”) from various species are propagated in common-garden environments. Scientists have leveraged these opportune experiments to examine the relative performance of the genets under identical environmental conditions. This research has revealed the remarkable intraspecific phenotypic diversity within these populations (Kiel et al. 2023). Notably, there is a wide range of sensitivity to ocean acidification (OA) in the branching species Acropora cervicornis. Some genets show no physiological impairment or reduction in growth rates, while others experience significant reductions, often exceeding 40%. This study seeks to investigate whether a biophysical, form-function relationship underlies this observed intraspecific OA sensitivity.
A coral’s shape is intimately linked to its physiological performance and, ultimately, survival. The colony geometry is molded by feedback loops with its environment and by competition with other species. Further, through acclimation strategies and phenotypic plasticity, fine-scale differences emerge across a continuous spectrum. These morphological diversities are likely to have ecologically relevant effects and arise from selective pressures from hydrodynamic stresses (Madin 2005) as well as optimizations for particle capture (Helmuth and Sebens 1993), photosynthetic performance (Hoogenboom et al. 2008; Kramer et al. 2022), and mass-transfer of physiologically-limiting metabolites, e.g., O2, pH, and dissolved inorganic carbon (Lesser et al. 1994; Mass et al. 2010). Therefore, the balance of these tradeoffs and competition among benthic organisms results in the tremendous morphological diversity observed on coral reefs (Chappell 1980).
Morphology can be described on a continuous scale, borrowing insights from geometry to integrate across morphological variation (Reichert et al. 2017; Zawada et al. 2019). Zawada et al. (2019) propose six metrics to constrain coral shape: sphericity and convexity (capturing volume compactness), packing and fractal dimension (capturing surface complexity), and the first moments of area and volume (capturing top-heaviness). Ultimately, the goal is to understand how each of these shape descriptors captures a coral’s physical interactions with its environment to spur physiological or ecological consequences. Of particular interest are the surface complexity metrics as these relate to the turbulence of water flow at the coral’s surface and influence mass flux (Stocking et al. 2018; Hossain and Staples 2020b, 2020a).
Corals with complex surfaces will experience increased turbulence and develop smaller microenvironments or diffusion boundary layers (DBL). These microenvironments arise from the localized attenuation of fluid flow at the coral’s surface. Advection is slowed or completely halted at these scales, and the diffusion rate dominates mass transfer (Patterson 1992; Shashar et al. 1996). Further, corals alter the chemistry of this entrapped volume through the changing balance of light-mediated carbon processes, including photosynthesis, respiration, calcification, and dissolution (Albright et al. 2015; Silbiger and Sorte 2018). During the day, a positive photosynthesis-to-respiration ratio increases dissolved oxygen (DO) and reduces carbon dioxide (CO2) concentrations. During the night, photosynthesis is halted, and there is a net decrease in DO and an increase in CO2. Between maximum net productivity shortly following solar noon and maximum net respiration shortly before sunrise, the pH and DO is a dynamic environment closely following the changing light exposure on the reef.
For example, Shashar et al. (1993) observed an oxygen microenvironment around the relatively smooth species Favia favus and measured oxygen concentrations greater than 300% air saturation in light and nearly complete anoxia in darkness. Similarly, Kühl et al. (1995) measured pH changes ± 0.5 units from bulk conditions under light and darkness within a 300 μm DBL. On the other hand, comparably rougher, branching species exhibited marginal to non-measureable metabolic changes in microenvironment oxygen and pH due to the elevated turbulence and mass flux (Kühl et al. 1995; Chan et al. 2016; Schoepf et al. 2018; Comeau et al. 2019). A conceptual figure combining the changing light conditions and various idealized surface roughnesses are summarized in Figure 1 below.
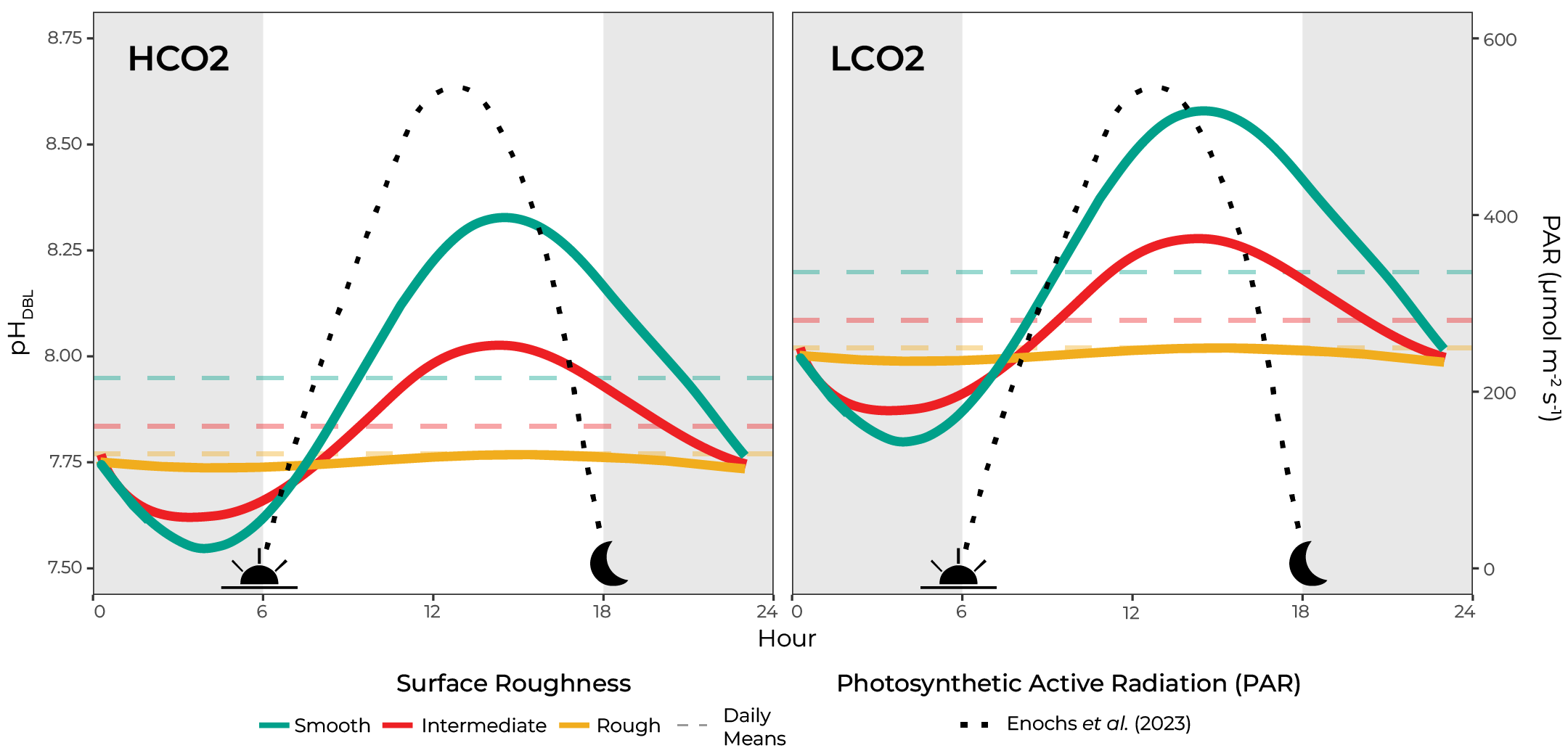
Figure 1. pH variability within the microenvironment (pHDBL) is proportional to the colony’s surface roughness, with smooth corals having higher day-time highs and lower night-time lows compared to comparably rougher morphologies.
The role of these microenvironments in modulating a coral’s response to OA ultimately relies on this discrete water volume influencing the calcifying fluid carbonate chemistry. The calcifying fluid (cf) is originally seawater, which has been partially closed off in cell junctions between the calicoblastic cells and the skeleton and is the ultimate source of carbon and ions for calcification (Allemand et al. 2011; Tambutté et al. 2011). Since the microenvironment envelops the coral colony and is the external seawater most proximal to the calcifying fluid, the microenvironment’s concentration likely plays a role in shaping the calcifying fluid carbonate chemistry. Further, the calcifying fluid aragonite saturation state ($\Omega_{cf}$) repeats the species-specific microenvironment pH pattern, with rough Acropora sp. having a reduced mean and diel variability than a comparably smoother Pocillopora damicornis (DeCarlo et al. 2019). Finally, the microenvironment is the center of the proton flux hypothesis, which postulates that OA reduces coral calcification due to the reduced diffusive flux of protons out of the calcifying fluid and across the microenvironment with increasing bulk seawater acidification (Jokiel 2011). Therefore, we are presented with two alternative hypotheses: 1) Smooth corals will elevate pH and experience daytime refugia, thereby maintaining elevated calcification rates (sensu Price et al. 2012); or 2) Rough corals will increase mass transfer and experience the highest night-time pH, thereby avoiding nightly dissolution
The goal of this experiment was to test which of these hypotheses applies to intraspecifc OA sensitivity for A. cervicornis, a generally sensitive species to OA.
Methods
This experiment exposed three A. cervicornis genets to either long-term low CO2 (LCO2; pH = 8.05) or high CO2 (HCO2; pH = 7.80) experimental aquarias, with replication of genets and treatments. Corals were collected from the University of Miami/Rescue a Reef coral nursery, and the experiment was run at the Experimental Reef Laboratory run by NOAA/University of Miami’s Cooperative Institute for Marine and Atmospheric Studies. A graphical overview of the experiment is presented in Figure 2.
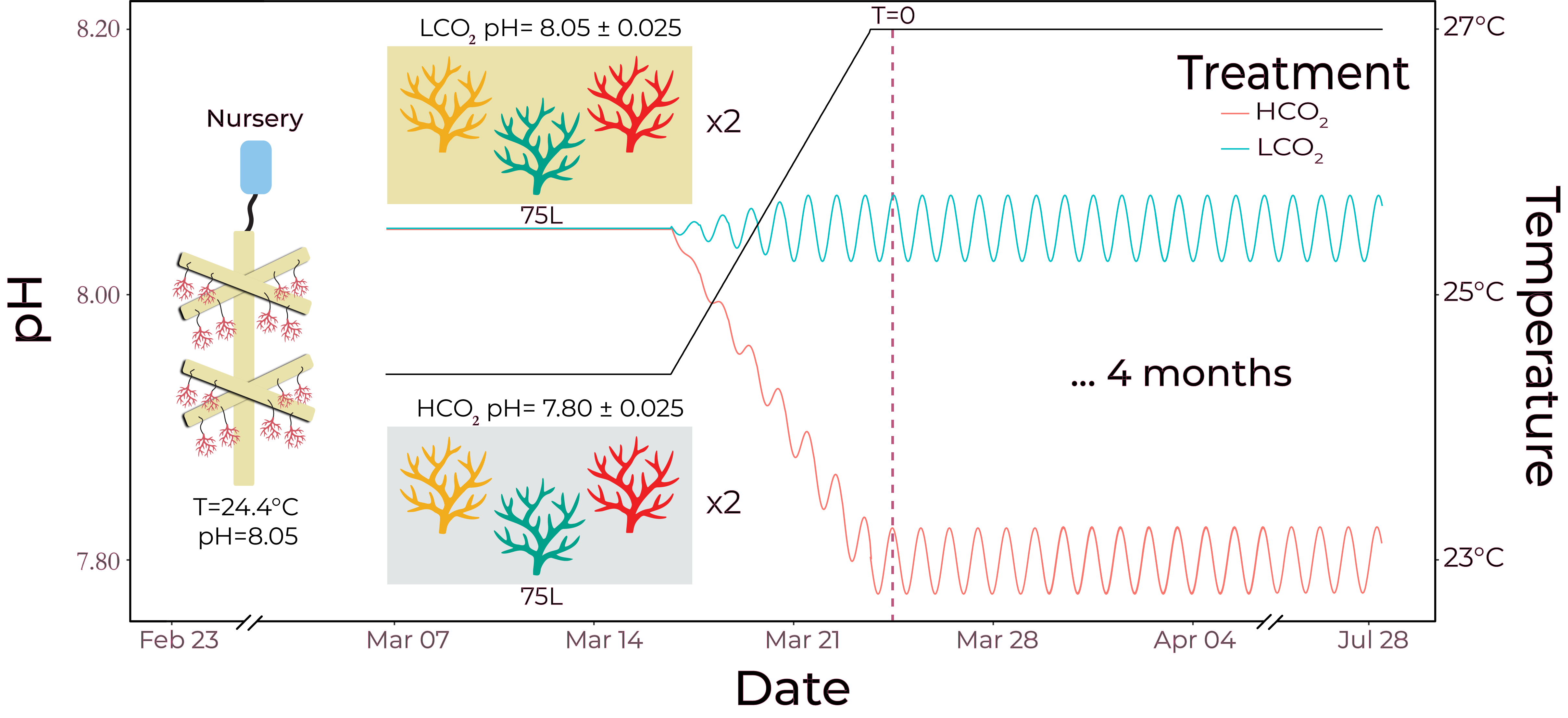
Figure 2. Experimental timeline, with programmed diel pH variability of 0.025 units, reflective of bulk water changes on a reef from long-term monitoring in the Florida Keys.
Growth rates were monitored weekly via the buoyant weight technique and were standardized to total surface area determined by a structured-light three-dimensional scanner. Reproduced 3D models were also used to calculate shape variables following methods outlined in Reichert et al. (2017) and Zawada et al. (2019). A principal components analysis was used to identify the shape variables that described the most variation within the data.
Following the four-month treatment, apical tips representing new skeletal growth exclusively grown during treatment were sectioned and prepared for geochemical analyses ($\delta^{11}B$) to serve as proxies for the pH of the calcifying fluid.
Eighteen randomly selected skeletons (n= 3/treatment/genet) were left intact following the growth phase to perform particle image velocimetry (PIV) and particle tracking velocimetry (PTV) analyses to calculate turbulence statistics around the coral colonies. Further, the 3D models were analyzed with computational fluid dynamics to calculate three-dimensional turbulence statistics and aid the PIV/PTV analyses. These data are still being collected and analyzed, and only a sample plot is produced.
Results
The three genets, collected from the same common-gardened nursery, exhibited a conserved morphology, with genet-specific fractal dimensions and packing, both of which describe surface complexity.
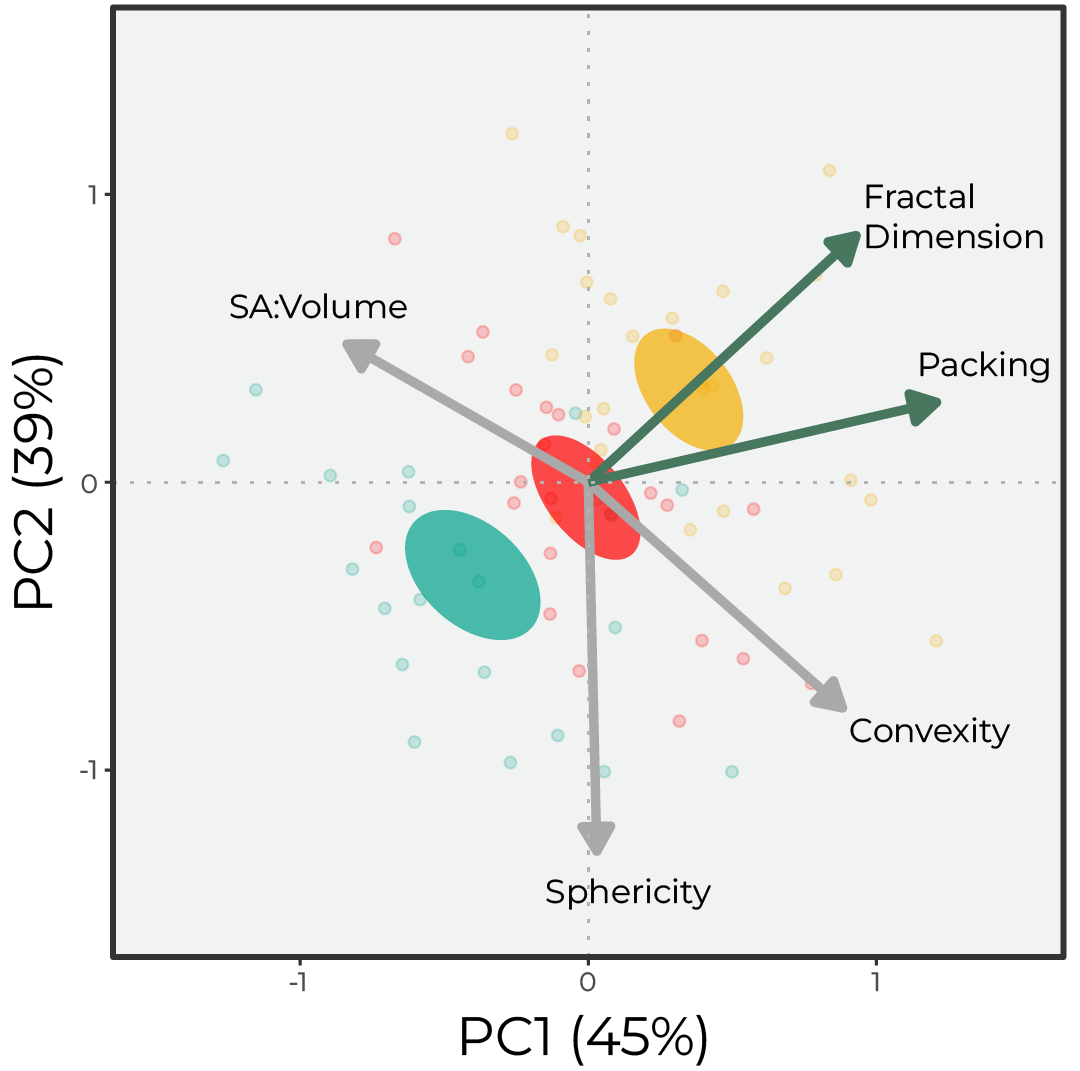
Figure 3. Principal Component Analysis (PCA) of the morphological variables highlights the three genet barycenters, which fall along the fractal dimension (FD) eigenvector. Eigen vectors are colored based on statistical significance between the three genets, alpha = 0.05.
There was an overall reduction in growth rates under the simulated OA (HCO2) group, but the genets differentially experienced OA stress.
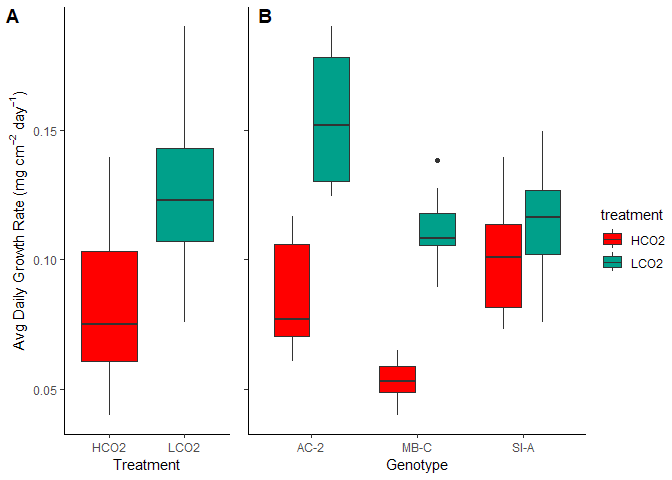
Figure 4. The three genets had differnetial sensitivity to OA stress
We can dive into the calcifying fluid pH with boron isotopes, and we see that calcifying fluid pH is almost inversely proportional to $pH_{cf}$. This falls in line with our conceptual plot where smoother corals experience higher day-time highs, driving the mean pH upwards since the change during the day is greater than the reduction at night (modeled at 3:1).
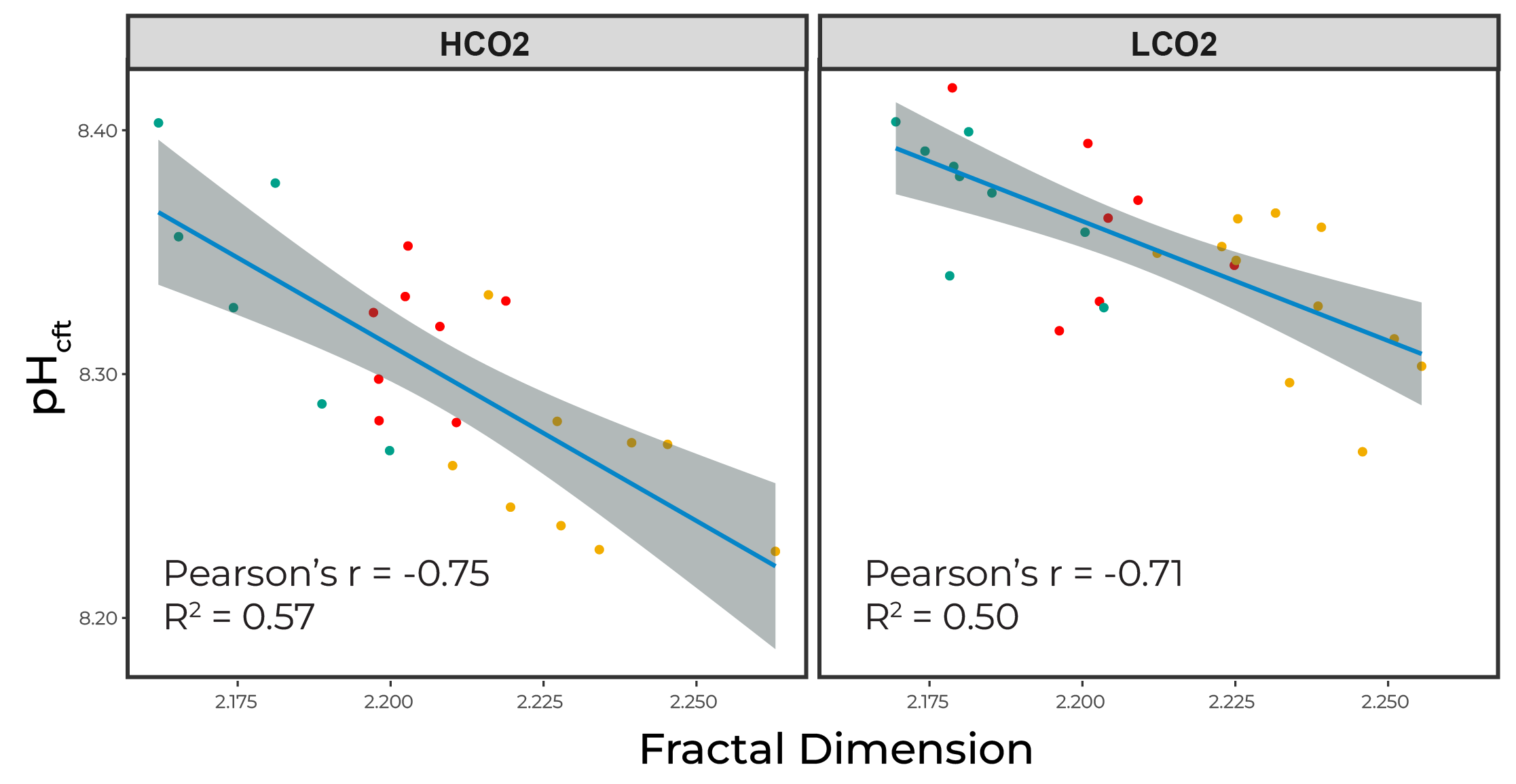
Figure 5. Calcifying fluid pH is inversely proportional to surface roughness
However, these $pH_{cf}$ are not driving OA sensitivites; rather corals with increased roughness experienced reduced sensitivity. This may be explained by the higher night-time lows in the rougher corals. Therefore, night-time dissolution may be driving OA sensitivity for A. cervicornis.
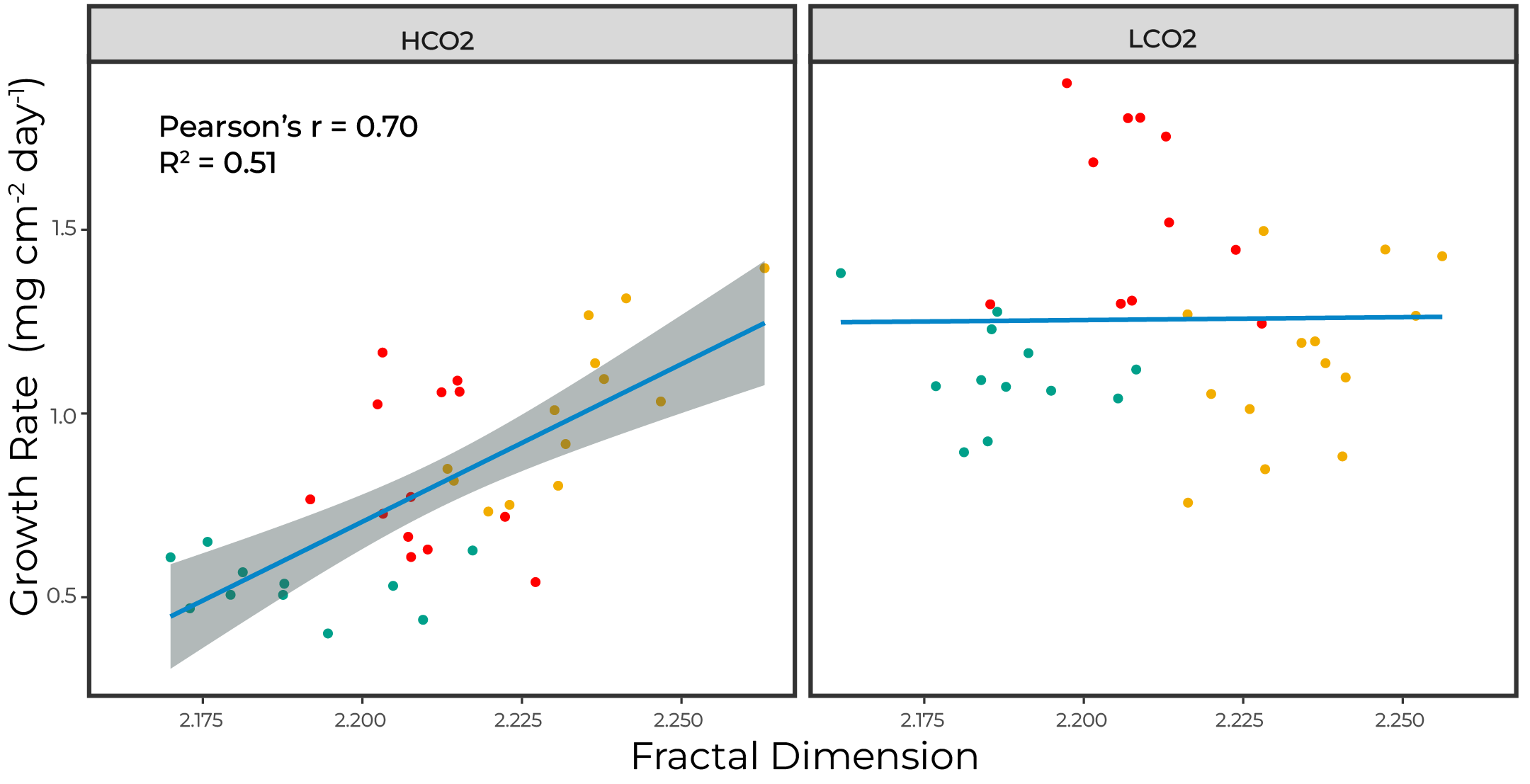
Figure 6. Growth rates scale with surface roughnes only under OA
Conclusions
These preliminary data suggest elevated calcifying fluid pH may not correlate with OA sensitivity in A. cervicornis. Rather, increased surface roughness reduces the night-time decrease in microenvironment pH and prevents nightly dissolution. This relationship is only present under simulated OA conditions and not under present-day scenarios. This is likely explained by the night-time lows in smooth corals exacerbating a pH threshold for the species when the average bulk pH is 7.8. It remains to be understood how this relationship plays out across multiple species, since A. cervicornis is a relatively sensitive species. Therefore, surface roughness is just one of the mechanisms that must be further investigated in addition to the molecular mechanisms of stress resistance.
Poster
References
Albright R, Benthuysen JA, Cantin NE, Caldeira K, Anthony KRN. 2015. Coral reef metabolism and carbon chemistry dynamics of a coral reef flat. Geophysical Research Letters. 42(10):3980–3988. https://doi.org/10.1002/2015GL063488
Allemand D, Tambutté E, Zoccola D, Tambutté S. 2011. Coral Calcification, Cells to Reefs. In: Dubinsky Z, Stambler N, editors. Coral reefs: An ecosystem in transition. Dordrecht: Springer; p. 119–150.
Chan NCS & Connolly SR. 2013. Sensitivity of coral calcification to ocean acidification: a meta-analysis. Global Change Biology. 19(1):282–290. https://doi.org/10.1111/gcb.12011
Chan NCS, Wangpraseurt D, Kühl M, Connolly SR. 2016. Flow and coral morphology control coral surface pH: Implications for the effects of ocean acidification. Frontiers in Marine Science. 3(FEB):1–11. https://doi.org/10.3389/fmars.2016.00010
Comeau S, Cornwall CE, Pupier CA, DeCarlo TM, Alessi C, Trehern R, McCulloch M. 2019. Flow-driven micro-scale pH variability affects the physiology of corals and coralline algae under ocean acidification. Scientific Reports. 9(1):1–12. https://doi.org/10.1038/s41598-019-49044-w
Chappell J. 1980. Coral morphology, diversity and reef growth. Nature. 286(5770):249–252. https://doi.org/10.1038/286249a0
DeCarlo TM, Ross CL, McCulloch M. 2019. Diurnal cycles of coral calcifying fluid aragonite saturation state. Marine Biology. 166(3):1–6. https://doi.org/10.1007/s00227-019-3468-6
Helmuth B, Sebens K. 1993. The influence of colony morphology and orientation to flow on particle capture by the scleractinian coral Agaricia agaricites (Linnaeus). Journal of Experimental Marine Biology and Ecology. 165(2):251–278. https://doi.org/10.1016/0022-0981(93)90109-2
Hoogenboom MO, Connolly SR, Anthony KRN. 2008. Interactions between morphological and physiological plasticity optimize energy acquisition in corals. Ecology. 89(4):1144–1154. https://doi.org/10.1890/07-1272.1
Hossain MM, Staples AE. 2020a. Mass transport and turbulent statistics within two branching coral colonies. Fluids. 5(3). https://doi.org/10.3390/fluids5030153
Hossain MM, Staples AE. 2020b. Effects of coral colony morphology on turbulent flow dynamics. PLOS ONE. 15(10):e0225676. https://doi.org/10.1371/journal.pone.0225676
Jokiel PL. 2011. Ocean acidification and control of reef coral calcification by boundary layer limitation of proton flux. Bulletin of Marine Science. 87(3):639–657. https://doi.org/10.5343/bms.2010.1107
Kiel PM, Formel N, Jankulak M, Baker AC, Cunning R, Gilliam DS, Kenkel CD, Langdon C, Lirman D, Lustic C, et al. 2023. Acropora Cervicornis Data Coordination Hub, an Open Access Database for Evaluating Genet Performance. Bulletin of Marine Science. 99(2):119–136. https://doi.org/10.5343/
Kramer N, Guan J, Chen S, Wangpraseurt D, Loya Y. 2022. Morpho-functional traits of the coral Stylophora pistillata enhance light capture for photosynthesis at mesophotic depths. Communications Biology. 5(1):861. https://doi.org/10.1038/s42003-022-03829-4
Kühl M, Cohen Y, Dalsgaard T, Jørgensen BB, Revsbech NP. 1995. Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and light. Marine Ecology Progress Series. 117(1–3):159–177. https://doi.org/10.3354/meps117159
Lesser MP, Weis VM, Patterson MR, Jokiel PL. 1994. Effects of morphology and water motion on carbon delivery and productivity in the reef coral, Pocillopora damicornis (Linnaeus): Diffusion barriers, inorganic carbon limitation, and biochemical plasticity. Journal of Experimental Marine Biology and Ecology. 178(2):153–179. https://doi.org/10.1016/0022-0981(94)90034-5
Madin JS. 2005. Mechanical limitations of reef corals during hydrodynamic disturbances. Coral Reefs. 24(4):630–635. https://doi.org/10.1007/s00338-005-0042-0
Mass T, Genin A, Shavit U, Grinstein M, Tchernov D. 2010. Flow enhances photosynthesis in marine benthic autotrophs by increasing the efflux of oxygen from the organism to the water. Proceedings of the National Academy of Sciences of the United States of America. 107(6):2527–2531. https://doi.org/10.1073/pnas.0912348107
Patterson MR. 1992. A chemical engineering view of cnidarian symbioses. American Zoologist. 32(4):566–582.
Price, N. N., Martz, T. R., Brainard, R. E. & Smith, J. E. Diel variability in seawater pH relates to calcification and benthic community structure on coral reefs. PloS one 7, e43843–e43843 (2012).
Reichert J, Backes AR, Schubert P, Wilke T. 2017. The power of 3D fractal dimensions for comparative shape and structural complexity analyses of irregularly shaped organisms. Methods in Ecology and Evolution. 8(12):1650–1658. https://doi.org/10.1111/2041-210X.12829
Schoepf V, Cornwall CE, Pfeifer SM, Carrion SA, Alessi C, Comeau S, McCulloch MT. 2018. Impacts of coral bleaching on pH and oxygen gradients across the coral concentration boundary layer: a microsensor study. Coral Reefs. 37(4):1169–1180. https://doi.org/10.1007/s00338-018-1726-6
Silbiger NJ, Sorte CJB. 2018. Biophysical feedbacks mediate carbonate chemistry in coastal ecosystems across spatiotemporal gradients. Sci Rep. 8(1):796. https://doi.org/10.1038/s41598-017-18736-6
Shashar N, Cohen Y, Loya Y. 1993. Extreme diel fluctuations of oxygen in diffusive boundary layers surrounding stony corals. Biological Bulletin. 185(3):455–461. https://doi.org/10.2307/1542485
Shashar N, Kinane S, Jokiel PL, Patterson MR. 1996. Hydromechanical boundary layers over a coral reef. Journal of Experimental Marine Biology and Ecology. 199(1):17–28. https://doi.org/10.1016/0022-0981(95)00156-5
Stocking JB, Laforsch C, Sigl R, Reidenbach MA. 2018. The role of turbulent hydrodynamics and surface morphology on heat and mass transfer in corals. Journal of the Royal Society Interface. 15(149). https://doi.org/10.1098/rsif.2018.0448
Suggett DJ, Guest J, Camp EF, Edwards A, Goergen L, Hein M, Humanes A, Levy JS, Montoya-Maya PH, Smith DJ, et al. 2024. Restoration as a meaningful aid to ecological recovery of coral reefs. npj Ocean Sustain. 3(1):1–4. https://doi.org/10.1038/s44183-024-00056-8
Tambutté S, Holcomb M, Ferrier-Pagès C, Reynaud S, Tambutté E, Zoccola D, Allemand D. 2011. Coral biomineralization: From the gene to the environment. Journal of Experimental Marine Biology and Ecology. 408(1–2):58–78. https://doi.org/10.1016/j.jembe.2011.07.026
Zawada KJA, Dornelas MA, Madin JS. 2019. Quantifying coral morphology. Coral Reefs. 38(6):1281–1292. https://doi.org/10.1007/s00338-019-01842-4